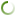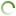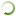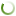Introduction
Foreign drugs have seized more than half of the Russian pharmaceutical market, virtually ousting Russia’s state–owned enterprises from the pharmaceutical industry and leaving Russian private companies with no other option but to function in a catching–up mode at best. The weak position of the Russian pharmaceutical industry in the domestic market generates major demographic risks to national security. The restoration of the industry is seen as a long–term governmental project, comparable in importance, for example, with the revival of civil aircraft or microelectronics. The paper continues the work (Gusev, Yurevich, 2023) in terms of analyzing the initial prerequisites and tactics of the anti–crisis mobilization model for the development of the Russian pharmaceutical industry.
As for the share of the pharmaceutical industry in its GDP, Russia, with an indicator of 0.4% (2020), lags behind Brazil (0.6% of GDP) and 12 times behind Switzerland (4.8% of GDP); this fact highlights significant potential for growth (Balatsky, Ekimova, 2023). We will not consider the scale of the industry in more detail here; rather, we will dwell on its internal structure and key performance indicators that help to see the available opportunities.
At the same time, we cannot say that federal authorities are not aware of Russia’s significant lack of independence in the field of drug production. In 2010, the Russian Government issued a list of drugs that must be produced by Russian companies. Since 2015, the Ministry of Industry and Trade of Russia prepared and has been implementing import substitution plans in the pharmaceutical industry. The state program “Development of the pharmaceutical and medical industry” [1] has been in operation for almost 10 years. However, despite the efforts undertaken by the government, including financial investments, the dependence on foreign drugs is not being reduced in favor of Russia’s national interests.
The aim of our work is to assess the effectiveness of the Russian pharmaceutical industry in order to understand the prospects for achieving drug self–sufficiency [2] and pharmaceutical leadership [3].
The effectiveness of Russian pharmaceutical production is considered in two related areas. The first one is a catching–up scenario of import substitution, when the task is to reproduce foreign drugs used in Russia [4]. Such import substitution has technological and economic specifics.
We should emphasize that the production of drugs within the country, even if there is a registration certificate in the name of a Russian organization, is not considered import substitution if such drugs are made on the basis of foreign pharmaceutical ingredients. In this case domestic companies perform secondary operations: dispensing, packaging, and final quality control. Thus, from the technological viewpoint, it is a mandatory requirement to master the production of active pharmaceutical ingredients (APIs) in Russia. In turn, this affects independence from foreign supplies in terms of production equipment, components and materials.
Another technical criterion for successful import substitution is the preservation of consumer characteristics in the reproduced drug (API) in terms of effectiveness and safety, which is determined by a set of factors (purity of the substances used, adequacy of the substitutes used, production quality, etc.). The characteristics may be compared by performing laboratory tests and surveying people who take the drugs.
From an economic point of view, full–fledged import substitution, including in terms of API production, assumes that there is no shortage of domestic products on the internal market, minimizes or excludes the import of finished drugs (APIs). If the production of an essential API is mastered by small businesses and is invisible on an industry scale, then the full–fledged import substitution scenario has not been implemented so far.
Under a catching–up scenario it becomes important to bridge the gap between Russian and foreign manufacturers. Methodologically, this effect needs comprehensive elaboration. One of the most accessible possibilities is to analyze the implementation of state import substitution plans.
The second area to promote effectiveness is to implement an outstripping development scenario for the Russian pharmaceutical industry. This scenario is an accelerator of catching–up import substitution. Outstripping refers to innovation activities in the development of original (new) drugs, i.e. unique products both for Russia and the world [5]. Stable and consistent innovation activity confirms pharmaceutical leadership. The number of original drugs developed by companies can be identified at the stage of clinical trials. The multiphase nature of clinical trials helps to carry out additional screening of original drugs, allowing only the most successful developments in terms of effectiveness and safety to be used in practical healthcare. Thus, the portfolio of developments for original drugs should be very extensive at the preclinical stage already.
In the pharmaceutical industry, catching–up development and outstripping development are not mutually exclusive. However, finding a balance between them in the field of allocating available resources and expected commercial efficiency becomes an independent management task at the level of Russian companies. In the current paradigm of public administration of the domestic industry, the tactics of catching–up development becomes a more understandable subject of regulation than the tactics of outstripping development associated with the complexity of goal setting, a longterm perspective and increased risks of failure. We should emphasize that in conditions of high import dependence, the problem of outstripping development can be solved on a very local scale, giving priority and the bulk of resources to the catching–up development scenario, which allows bridging the product–related and technological gap.
Flaws in finding the optimal combination of catching–up and outstripping modes of functioning for the pharmaceutical industry will affect end users in the following ways: if priority is given to outstripping development, then widely used drugs will not be available or will be hard to purchase; on the other hand, under a lingering catching–up scenario that does not end within a reasonable time frame with a parity at least according to the list of key product positions, the effectiveness of replicated drugs will be comparatively lower, in addition to the lack of obvious opportunities for their improvement.
As for the methodological novelty of our work, we should note the tested approaches in the analytical use of industry sources of primary information about products, without which it is not possible to identify and analyze the specific results of the catching–up and outstripping mode of pharmaceutical industry development (the State Register of Medicines, the Register of Authorized Clinical Trials of Medicines, state sectoral import substitution plans).
The novelty of the research findings consists in assessing the success of the implementation of catching–up and outstripping vectors of development of the Russian pharmaceutical industry, as well as substantiating the need for a new paradigm for organizing the pharmaceutical industry to replace its current configuration, which has a range of administrative, structural, production and marketing flaws (decentralization of sectoral public administration, unreasonably minimized public pharmaceutical sector, fragmentation of the private sector, critical dependence on imported pharmaceutical ingredients, stable dominance of Big Pharma companies in the Russian market).
The long–term effectiveness of public administration in the pharmaceutical industry and, accordingly, industry performance can be assessed with a considerable degree of skepticism if we look at the provisions of the Strategy for the Development of the Pharmaceutical Industry of the Russian Federation for the period through to 2030 [6]. According to the document, issued by the Ministry of Industry and Trade of Russia, the vector of development of the industry will ideologically repeat the past decade, which was not marked by major progress.
Before analyzing the pharmaceutical effectiveness of the Russian industry, let us look at the main economic indicators of Big Pharma companies and leading Russian organizations. This will allow us to compare resources and assess competitive capabilities of the parties.
Comparative research activity of pharmaceutical companies
With the exception of the generic drugs segment, the global pharmaceutical market establishes high barriers for new entrants; these barriers are related more to the accumulation of technological resources. As a result, real market power is concentrated in the hands of several major corporations, which are often called “Big Pharma” (Dosi et al., 2023). The pharmaceutical industry, in fact, is controlled by a narrow circle of foreign companies who take great pains to protect their production technologies and control sales flows.
The generic drugs market is becoming more accessible for countries with an emerging pharmaceutical industry, while achievements in the development and sale of original drugs are rather exceptions to the rules. The reason lies in the increasing cost of creating such drugs. Against the background of steady growth in R&D, preclinical and clinical trials costs, for many years there has been a trend toward a decrease in the number of original drugs approved by relevant agencies (U.S. Food and Drug Administration, etc.) (Paul et al., 2010). For example, in the 2000s the average cost of bringing drugs based on a molecular compound to the market was approximately 1.8 billion USD; almost 10 years later the sum reached 2.8 billion USD (DiMasi et al., 2016), and by 2020 – 6.2 billion USD (Schuhmacher et al., 2023). Despite the fact that the figures obtained are not fully comparable (different samples of companies, calculation methods, etc.), the trend toward an increase in the cost of creating original drugs is highlighted consistently in many works (Pammolli et al., 2011; Meier et al., 2013; Kruse et al., 2014; Pammolli et al., 2020). Moreover, by 2020, seven out of the 16 Big Pharma companies surveyed had negative R&D performance (the ratio of profits from new drugs to R&D costs) (Schuhmacher et al., 2023).
We should also note metamorphoses in the development of original drugs by Big Pharma companies. For instance, in 2009–2018, the top 10 pharmaceutical companies registered more than half of new drugs in just one year, and in 2017–2018, even the top 30 could not obtain half of the licenses [7]. Startups and medium–sized companies (with annual revenues of up to 1 billion USD) are gradually becoming sources of innovative drugs (at least at the initial stages of development). But pharmaceutical giants are using another advantage to maintain their positions. Huge internal capital and almost unlimited borrowing opportunities allow them to absorb competitors: in recent years, about 80% of such transactions have been completed by representatives of Big Pharma [8] (HBM, 2023). Thus, the risks associated with huge investments in the development of new drugs are balanced (Bereznoy, 2022; Redit, 2022; Keenan et al., 2023); this, in particular, allowed the abovementioned corporations with negative R&D performance to show positive financial results (Schuhmacher et al., 2023).
Big Pharma can be ousted from the national market, but this requires proactive approach on the part of governments. In addition to countries with a developing pharmaceutical industry, this task has been set even at the level of the European Union: a course toward strengthening industrial and technological sovereignty in the field of vaccines and other drugs has been approved (Groshkova et al., 2021). This was stimulated, among other things, by critical difficulties emerging in the vaccination campaign during the COVID–19 pandemic due to dependence on supplies from manufacturers located outside the European Union (Crespi et al., 2021).
In most cases, foreign drugs available on the Russian market are connected with large multinational pharmaceutical companies. Attempts to oust them from the Russian market as a result of competitive administrative struggle will inevitably lead to confrontation not only with the companies themselves, but also with the relevant countries.
Success in this struggle can be achieved when domestic drugs are at least comparable to foreign ones in terms of their effectiveness and safety. This places high, even global, demands on Russian developers, resources, and infrastructure. For example, it will be rather difficult to address complex pharmaceutical tasks in conditions worse than those of our competitors.
Table 1 shows economic indicators for some Big Pharma companies that have occupied the Russian market with multibillion–dollar sales, and for leading private Russian organizations.
Table 1. Commercial and innovative activity of Big Pharma and the most prominent Russian pharmaceutical companies, 2022, billion USD
|
No. |
Pharmaceutical company |
Country |
Profit (year) |
R&D expenditures (year) |
|
1 |
Pfizer |
USA |
100,33 |
11,43 |
|
2 |
Johnson & Johnson |
USA |
94,94 |
14,6 |
|
3 |
Roche |
Switzerland |
66,26 |
14,71 |
|
4 |
Merck & Co |
Germany |
59,28 |
13,55 |
|
5 |
AbbVie |
USA |
58,05 |
6,51 |
|
6 |
Bayer |
Germany |
53,459 |
6,924 |
|
7 |
Novartis |
USA |
50,55 |
10,00 |
|
8 |
Bristol–Myers Squibb |
USA |
46,16 |
9,51 |
|
9 |
Sanofi |
France |
45,22 |
7,06 |
|
10 |
AstraZeneca |
UK – Sweden |
44,35 |
9,76 |
|
11 |
Abbott |
USA |
43,653 |
2,888 |
|
12 |
GlaxoSmithKline |
UK |
36,271 |
6,788 |
|
13 |
Takeda |
Japan |
31,764 |
4,682 |
|
14 |
Eli Lilly |
USA |
28,54 |
7,19 |
|
15 |
Gilead Sciences |
USA |
27,281 |
4,977 |
|
16 |
Amgen |
USA |
26,323 |
4,434 |
|
17 |
Novo Nordisk |
Denmark |
25,057 |
3,405 |
|
18 |
Boehringer Ingelheim |
Germany |
25,555 |
5,341 |
|
19 |
Regeneron Pharmaceuticals |
USA |
12,173 |
3,593 |
|
20 |
Biogen |
USA |
10,173 |
2,231 |
|
21 |
R–Pharm JSC |
Russia |
2,489* |
0,003* |
|
22 |
Pharmstandard JSC |
Russia |
1,971 |
Data not available |
|
23 |
Generium JSC |
Russia |
1,609* |
Data not available |
|
24 |
Biocad JSC |
Russia |
1,262* |
Data not available |
|
25 |
Otisipharm JSC |
Russia |
0,646 |
Data not available |
|
* Data as of 2021. |
||||
First of all, we observe huge disparity in the scale of business of Russian and foreign companies by several orders of magnitude in favor of the latter.
The situation in the competition will be extremely unfavorable: “one small company against many large ones”. Moreover, we note considerable difference in the values of research activity indicators: the share of R&D costs in the profit. Among Big Pharma companies, this figure was at least 10%, with Abbot being the only exception: 6.62% of 43.65 billion USD in 2022. Regeneron Pharmaceuticals was leader in research activity in 2022: 29.5% of 12.2 billion USD. Against this background, the annual R&D expenses of the Russian pharmaceutical leader (R–Pharm JSC) in the amount of 241 million rubles (0.13% of annual profit) do not indicate a desire to compete with anyone in at least one commodity position. By the way, in the pre–pandemic period, Russian pharmaceutical companies spent on average 1–2% of profit on R&D (Komarova, Petrov, 2016), but these are, most likely, optimistic estimates.
Effectiveness in import substitution
The restoration of domestic production of essential drugs in Russia is a strategic task that is being addressed, but that has not been solved for decades. Various quantitative assessments of the success of import substitution processes in the Russian economy in general and the pharmaceutical industry in particular indicate in favor of strengthening the positions of Russian manufacturers (Litvinova et al., 2019). But at the same time, the question of the necessary/sufficient level of production sovereignty for a particular market or product group is often left out. The pharmaceutical industry is usually regarded as one of the most vulnerable and dependent on external supplies of raw materials and equipment; nevertheless, according to some expert assessments, the transition to an import–substituting model, launched in 2014, proved quite successful, a critical mass of high–tech manufacturers emerged on the territory of Russia, etc. (for example, Dorzhieva, 2022).
An insufficiently active participation of the government is often considered one of the reasons hindering import substitution. It is noted that the role of the government, among other things (for example, subsidies, tax incentives, direct and indirect support for R&D, establishing a network of pharmaceutical clusters, etc. (Kotlyarova et al., 2017; Krestyaninov, 2018; Dorzhieva, 2023)), should consist in creating “anchor demand” for innovative drugs of Russian production (Mamedyarov, 2017). And in this context, the government is represented not only by federal agencies, but also by the authorities of RF constituent entities, which are strongly interested in the development of regional industry (Gulin et al., 2015).
Real assessment of import substitution processes is hampered by the lack of reliable and time– comparable information. The analytical reports of DSM Group, which are considered one of the most reliable sources of quantitative information about the Russian pharmaceutical market, also provide only an approximate idea of the displacement of foreign competitors (Fig. 1). In particular, since 2020, localized drugs have been taken into account in the group of domestic drugs, and annual market estimates have been replaced by monthly ones. Nevertheless, in the period from 2013 to 2022, the market share of drugs of domestic production, apparently, increased by more than 10 p.p. According to other data, Russian drugs occupied almost two thirds of the retail market back in 2020 (Abdikeev, 2022).
.png)
RF Government Resolution 1141–r, dated July 6, 2010 approved and further updated the list of essential/strategically important drugs, the production of which should be established on the territory of the Russian Federation. As amended on August 1, 2020, this list includes 214 items. Nevertheless, the governmental document does not contain any deadlines for the development of production (in case of its absence), or production volumes, or dosage forms. These issues are left to the Ministry of Industry and Trade of Russia.
After the introduction of anti–Russian sanctions in 2014, Order 656 of the Ministry of Industry and Trade of the Russian Federation, dated March 31, 2015 approved an industry action plan for import substitution in the pharmaceutical industry, designed for the period up to 2020. This plan was updated only once (September 4, 2018). Currently, the document contains a list of 602 drugs with different values of two indicators: “actual indicator of the share of imports before the implementation of the project” and “maximum planned indicators of the share of imports until 2020”.
The logic of the first “five–year plan” of pharmaceutical import substitution has significant flaws.
First, the term “medicinal product” combines a medicinal product and a pharmaceutical substance. Hence, it is not at all clear which category each of the 602 positions belongs to. Drugs are not grouped by any characteristics and, thus, are not systematized by application areas. The dosage forms have not been identified.
Second, the import substitution scenario, which consists in the development of analogues to foreign drugs, is combined with the scenario of loading additional capacities inside Russia in conditions of production development. Thus, 340 drugs belong to the production scenario, especially 104 items with an actual import share of no more than 10%; and 262 drugs with an import share of 100% belong to the research scenario (Fig. 2).
.png)
Third, the management parameter “maximum planned indicators of the share of imports until 2020” is very cunning, since it allows for a complete lack of progress in import substitution. From an administrative point of view, the applied parameter “maximum planned indicators of the share of imports until 2020” turns the lack of progress into a routine workflow.
For many import–substituting positions, goal setting cannot be interpreted in any way at all. In particular, for 37 drugs, the planned value of the import share for 2020 coincides with the value of the import share at the starting point (2015) – 100%.
Fourth, quantitative targets related to imports are very problematic in accounting and allow for the manipulation of figures. On the one hand, it has not been determined which values – monetary or natural – will be used in estimating import volumes.
On the other hand, the size of imports may be unstable, especially in the five–year perspective, due to the development of production technologies, the appearance of substitute goods, including those of foreign origin; changes in the economic environment, strategies of importers and exporters, and for other reasons.
We should note that the official results of implementing the plan of the Ministry of Industry and Trade of Russia for 2015–2020 have not been announced. Technically, the results of import substitution can be verified using the State Register of Medicines (GRLS) by checking manufacturers for 602 items on the list of drugs and (or) pharmaceutical ingredients, which means a tremendous amount of work that may not necessarily provide unambiguous answers, taking into account the technological and economic aspects discussed above. In particular, this information should be supplemented with information that is not publicly available and that concerns the actual volumes and degree of localization of target drugs production, comparability with foreign analogues in terms of effectiveness and safety, physical and cost sales volumes in the Russian market.
A random check of the top ten drugs listed in Order 656 of the RF Ministry of Industry and Trade, dated March 31, 2015 shows that as of August 2023 no traces of even formal success in import substitution were found in GRLS for a number of drugs / active pharmaceutical ingredients (Tab. 2).
Table 2. Information about drug manufacturers
|
No. |
Name of drug |
Project implementation timeframe |
Actual import share before project implementation, % |
Maximum planned indicators of import share until 2020, % |
Availability of Russian manufacturer (according to GRLS data)* |
|
|
API |
MP** |
|||||
|
1 |
Glatiramer acetate |
2015–2020 |
100 |
50 |
+ |
– |
|
2 |
Trastuzumab |
2015–2020 |
100 |
50 |
– |
– |
|
3 |
Bevacizumab |
2015–2020 |
100 |
50 |
+ |
– |
|
4 |
Infliximab |
2015–2020 |
100 |
50 |
+ |
+ |
|
5 |
Abacavir + lamivudine |
2015–2020 |
100 |
10 |
– |
– |
|
6 |
Atazanavir |
2015–2018 |
100 |
10 |
+ |
+ |
|
7 |
Octocog alpha |
2015–2020 |
100 |
50 |
+ |
– |
|
8 |
Budesonide + formoterol |
2015–2020 |
100 |
50 |
– |
– |
|
9 |
Raltegravir |
2015–2020 |
100 |
100 |
+ |
– |
|
10 |
Poractant alfa |
2015–2020 |
100 |
100 |
– |
– |
|
API – active pharmaceutical ingredient; |
||||||
Thus, for four out of ten drugs, it was not possible to find evidence of import substitution:
— trastuzumab (antitumor agent);
— abacavir + lamivudine (a drug for the treatment of HIV infection in combinations);
— budesonide + formoterol (a combined bronchodilator);
— poractant alfa (a remedy for the treatment / prevention of respiratory distress syndrome (RDS) in premature infants).
Chronologically, having completed the first five–year import substitution plan with quite limited success, the Russian Ministry of Industry and Trade adopted a second and more focused plan for the period up to 2024 (Order 2681 of the Ministry of Industry and Trade of Russia, dated July 20, 2021). The content of the document has undergone significant changes. The new list contains 65 items, including 38 drugs and 27 active pharmaceutical ingredients to them.
The annual demand for 27 active pharmaceutical ingredients subject to import substitution was estimated by the Ministry of Industry and Trade of Russia at a rather modest amount: 3.15 billion rubles. The volume of demand for APIs in physical terms is defined with great variation: from incomplete grams (buprenorphine (semi–synthetic opioid, analgesic): 0.1 g per year) to several tons (valproic acid (antiepileptic): 7.8 tons per year). According to RNC Pharma, in 2021, the total volume of APIs imports to Russia amounted to 195.4 billion rubles, and in physical terms – 15.8 thousand tons [9]. In the context of this information, the goal of the RF Ministry of Industry and Trade in the field of import substitution of APIs looks very unambitious, since it covers 1.6% of the country’s needs in value and a very negligible amount in kind.
For all items subject to import substitution, the values of the initial and target indicators coincide, and now they are in no way related to imports:
— the share of domestic products produced according to the full production cycle before the implementation of the import substitution plan: 0%;
— the share of domestic products produced according to the full production cycle, until 2024: 100%.
Thus, in the second plan of import substitution, the formal substantive mistakes of the predecessor have been largely eliminated; however, new ones, no less severe in their consequences, have been made in terms of slowing down the pace.
We should pay attention to the intersection of the first and second plans of the Ministry of Industry and Trade of the Russian Federation for import substitution: 32 of the 38 items of the second plan actually continue the agenda laid down in 2015, which indicates that the task regarding the relevant drugs is unresolved. Only six items are named as the new drugs (APIs) that are included in the targeted import substitution for the period up to 2024: buprenorphine, dapagliflozin, dimethyl fumarate, cabazitaxel, omalizumab, tenecteplase.
Comparing the lists of drugs included in the import substitution plans of the RF Ministry of Industry and Trade with the list of drugs established by the RF Government, we can find a number of intersections and “blind” zones. In general, the plans of the RF Ministry of Industry and Trade still do not cover 29 drugs listed in RF Government Resolution 1141–r, dated July 6, 2010 (13.5% of the total number of items), which makes the prospects for their production in Russia uncertain, and the tasks set by the government – not accepted for execution [10].
Problems of import substitution regarding socially significant drugs
Under the current regulatory framework, vital and essential drugs (VEDs) made up a special group; their list is established by RF Government Resolution 2406–r, dated October 12, 2019 (827 drugs, as amended on December 24, 2022) [11]. We should note that the focus of the governmental policy on the domestic production of drugs in Resolution 1141–r, dated July 6, 2010 seems to be minimalist, since it is four times less than the composition of VEDs.
The introduction of anti–Russian sanctions led to the formation of another drug group besides VEDs: drugs that are potentially in short supply. Their composition is determined by the interdepartmental commission of the Ministry of Health of the Russian Federation and, as of February 2023, includes 77 items [12].
The comparison of the two lists forms the most problematic segment – 51 VEDs in short supply, which is the subject of priority consideration from the point of view of the need for import substitution. The comparative analysis shows that only 43 (out of 51) drugs appeared in the import substitution plans of the RF Ministry of Industry and Trade (2015–2020). The remaining eight items are very diverse in areas of application; moreover, they are not produced in Russia and are not even registered in GRLS on behalf of foreign companies (Tab. 3).
Table 3. VEDs in short supply that were not included in the import substitution program
|
No. |
Name of drug |
Description |
|
1 |
Nivolumab |
Antitumor monoclonal antibody |
|
2 |
Dulaglutide |
Used for the treatment of type 2 diabetes mellitus |
|
3 |
Pembrolizumab |
Immuno–oncological drug for the treatment of malignant tumors |
|
4 |
Sapropterin |
Used for the treatment of hereditary fermentopathies |
|
5 |
Potassium acetate + Calcium acetate + magnesium acetate + sodium acetate + sodium chloride |
Rehydrating agent |
|
6 |
Naloxone + oxycodone |
Opioid narcotic analgesic |
|
7 |
Pegaspargaza |
A remedy for the treatment of acute lymphoblastic leukemia |
|
8 |
Peritoneal dialysis solution (CAPD/DPCA 2, 3, 4, 17, 18, 19) |
Used for the treatment of kidney failure |
|
Source: own compilation. |
||
Let us consider the information about manufacturers of active pharmaceutical ingredients for 43 drugs from the “VEDs in short supply” group, which are registered in GRLS in various dosage forms:
6 drugs are produced only by Russian manufacturers;
25 drugs are produced by Russian and foreign manufacturers;
12 drugs are produced only by foreign manufacturers [13].
This analytical information expands the knowledge about the effectiveness of the import substitution program of the RF Ministry of Industry and Trade on the example of another random sample of drugs. We can confirm that at present import substitution has not been implemented for 12 drugs for which APIs are not produced in Russia.
Despite the fact that 25 out of 43 drugs have not only foreign manufacturers of APIs, but are also produced by Russian firms, some items are in the area of unstable import substitution. Instability is characterized, first of all, by the presence of only one Russian manufacturer of APIs. A similar situation is observed with respect to APIs for a number of drugs produced only by Russian companies (Tab. 4).
Table 4. Drugs with unstable import substitution
|
No. |
Name of drug |
Name of the only domestic manufacturer of API |
Information about the manufacturer’s profit, billion rubles (year) |
|
1 |
Amoxicillin (antibiotic)* |
Biokhimik JSC, Saransk |
0,025 (2021) |
|
2 |
Acetylsalicylic acid (aspirin)* |
Irbitskii khimfarmzavod JSC, Irbit |
2,30 (2021) |
|
3 |
Dextrose (glucose)* |
Medsintez Plant, Yekaterinburg |
3,65 (2021) |
|
4 |
Retinol (vitamin A, antioxidant)** |
JSC Pharmaceutical Factory of Saint Petersburg, Saint Petersburg |
0,164 (2021) |
|
5 |
Prednisolone (hormonal anti–inflammatory, anti–allergic drug)** |
RENEWAL, Novosibirsk |
9,03 (2021) |
|
* Produced by Russian and foreign manufacturers. |
|||
Industrial monopolism, including dwarf monopolism, is not the best situation for a country in the field of APIs production for many reasons. According to the form of ownership, monopolistic companies do not belong to the public sector of the economy, their product portfolio is independent. There is no backup in case of corporate changes in production; neither is there a possibility, if necessary, to increase production promptly. In addition, the scale of activities of individual manufacturing companies is so small that we can say that the import substitution of VEDs in short supply has actually failed (see “Amoxicillin” and “Retinol” in Tab. 4).
Considering the adoption of RF Government Resolution 1141–r, dated July 6, 2010 as the official launch of import substitution, we can conclude that achievements in this area have not been evident. Under the current import substitution paradigm, the implementation of a catching–up scenario can take an infinitely long time in the absence of a noticeable bridging of the gap. It is also necessary to take into account the aggravating circumstances of the pharmaceutical chase: the composition of foreign drugs on the Russian market is being updated at a pace set by world leaders, and the developments themselves are objectively becoming more complicated for reproduction.
There is a political slogan that the most successful strategy for a laggard is to avoid the “chase” and immediately move on to outstripping, that is, creating exclusively new products that no one in the world possesses. The work (Balatsky, Ekimova, 2019), using the methodology of expanded innovation and technology matrices, shows a combination of labor productivity and research and development costs by country (both macroindicators are given relative to the United States). Using the example of many countries, the authors prove that, in general, relative innovation activity does not exceed relative labor productivity, that is, the above slogan does not work.
At the same time, there is a very narrow circle of small countries (Israel, South Korea) that implement a proactive innovation strategy in short time periods when increased research and development costs become disproportionately higher than relative labor productivity (Balatsky, Ekimova, 2019). Potentially, due to this breakthrough, leadership in a single high–tech industry can be achieved for some time.
The modern market for generated pharmaceutical innovations is limited only by the objective parameters of the planet. Under these conditions, even very large investments in the development of drugs are fully recouped in a reasonable time when the clinical effectiveness of the development is achieved. Thus, the specifics of the pharmaceutical industry leave chances for individual countries to achieve industry leadership if they really strive for it and apply resources.
Let us consider the scenario of advanced development of the Russian pharmaceutical industry through the prism of clinical trials of original drugs conducted in the country.
Effectiveness issues in pharmaceutical innovation
Clinical trials of original drugs are the spearhead of pharmaceutical leadership. Trials test creativity and innovation of medical science and the pharmaceutical industry in terms of developing new drugs (Wouters, 2020; Vargason et al., 2021). The greater the number of original drugs, the more powerful is the national pharmaceutical “machine”. However, not every country has the right and opportunity to develop original drugs, like they develop other high–tech industries (Weigmann, 2015; Park et al., 2021).
The profile of clinical trials of drugs in the Russian Federation, initiated in the period 2009– 2022, is shown in Table 5. We should note that the number of clinical trials in its bulk exceeds the number of drugs as products under development (including as a result of the introduction of new dosage forms for previously registered drugs, expansion of indications for their use).
Table 5. Permits issued for clinical trials of medicinal products, 2009–2022, units
|
Year |
Total |
IMCTs |
Foreign sponsors |
Russian sponsors |
||
|
Local CIs |
Bioequivalence |
Local CIs |
Bioequivalence |
|||
|
2009 |
577 |
348 |
32 |
8 |
112 |
77 |
|
2010 |
482 |
246 |
30 |
6 |
123 |
77 |
|
2011 |
567 |
370 |
35 |
19 |
80 |
63 |
|
2012 |
915 |
369 |
62 |
107 |
165 |
212 |
|
2013 |
791 |
334 |
68 |
110 |
124 |
155 |
|
2014 |
750 |
282 |
62 |
123 |
142 |
141 |
|
2015 |
804 |
289 |
52 |
143 |
167 |
153 |
|
2016 |
897 |
302 |
82 |
146 |
197 |
170 |
|
2017 |
700 |
281 |
48 |
71 |
149 |
151 |
|
2018 |
653 |
287 |
26 |
69 |
130 |
141 |
|
2019 |
746 |
313 |
35 |
80 |
155 |
163 |
|
2020 |
734 |
322 |
18 |
56 |
139 |
199 |
|
2021 |
908 |
367 |
36 |
87 |
133 |
285 |
|
2022 |
740 |
124 |
16 |
71 |
162 |
367 |
|
IMCTs – international multicenter clinical trials; CIs – clinical trials. |
||||||
The data in Table 5 allow us to draw the following conclusions.
First, for almost the entire observation period (2009–2021), the share of clinical trials of drugs associated with foreign companies, including IMCTs, exceeded 50%. In some years, this value was more than 60% (2009, 2011, 2013–2015). The maximum was reached in 2011 (74%). This fact testifies not only to the openness of this industry, but also to the weakness of Russian companies amid foreign players.
Second, the territory of the Russian Federation turned out to be given over to international multicenter clinical trials, which recruited a certain number of Russian personnel with medical education. The share of IMCTs in the total volume of initiated clinical trials steadily amounted to 40% (maximum in 2011 – 65%), with the exception of 2022. Strictly speaking, the involvement of the Russian Federation in IMCTs does not give any advantages to either domestic developments or domestic manufacturers. Compared, for example, with Ukraine and Georgia, where uncontrolled biological laboratories of foreign countries are located, the scenario under which many IMCTs are launched on the territory of the Russian Federation, at least under governmental control, looks like a more sparing form of colonial model of the pharmaceutical industry.
Third, since 2012, we should note a sharp increase in the number of bioequivalence clinical trials of drugs funded by Russian companies. This vector for the creation of domestic drugs reproducing foreign analogues is synchronized with RF Government Resolution 1141–r, dated July 6, 2010 and emphasizes the tactics of following in the wake of foreign pharmaceutical developments.
In 2022, this strategy only intensified due to the withdrawal of some foreign companies from Russia, the cessation or restriction of sales of certain original drugs of foreign origin. Currently, counting on reproduced drugs is seen as having no alternative. The relatively quiet time to conduct own developments has been lost.
Fourth, the 2012–2016 period with a large number of bioequivalence clinical trials with foreign sponsorship highlights a certain stage in the history of the Russian industry. It can be assumed that it was about positioning the Russian Federation as a territory where some foreign companies clinically tested copied products of other pharmaceutical companies. Since 2017, activity in this area has decreased markedly, but has not disappeared entirely.
Let us consider original drugs once again. We should note that this term was legislated only in December 2019. This allows us to identify the innovativeness of Russian pharmaceutical production at the institutional level (Tab. 6).
Table 6. Permits issued for clinical trials of original drugs, 2020–2022, units
|
Year |
Total number of original drugs in initiated clinical trials |
Including |
|
|
with foreign sponsorship |
with Russian sponsorship* |
||
|
2020 |
15 |
5 |
10 |
|
2021 |
8 |
1 |
7 |
|
2022 |
20 |
1 |
19 |
|
* Original biological drugs. |
|||
Despite the small number of original biological drugs that can be classified as high–tech, their composition and developers are not listed in ACTO analytical materials. We emphasize that the classification of drugs as original is the result of an expert assessment by ACTO authors. Strictly speaking, the number of original drugs of Russian developers for which clinical trials have been initiated is recommended to be tacitly considered at the official level as a key industry performance indicator.
Trusting the expert assessment of ACTO, let us compare the number of original drugs being developed that have entered the stage of clinical trials with the economic parameters of the Russian pharmaceutical sector, including private and state–owned companies. With a market share of domestic drugs of more than 1 trillion rubles per year, the number of original drug developments seems to be very low.
The stage of clinical trials is quite risky from the point of view of the further life cycle of a drug. Taking into account clinical trial failures, the number of original drugs that have reached the end user tends to zero. Thus, it is possible to talk about acceptable innovativeness of domestic companies on the condition that the number of original drugs they have developed and that have reached the stage of clinical trial will be several hundred per year, that is, at least an order of magnitude higher than the current level.
Is it possible to achieve such effectiveness under the current situation in the Russian segment of the pharmaceutical industry? Most likely, it is not, due to the following reasons.
First, the dominant private business, striving for profit, tends to adhere to the strategy of reproduction of drugs and focus on their internal sales, rather than undertake investments in highrisk projects to create new products. It is not capable and not ready to enter the international market and compete with external players, since it does not even dominate the domestic market. Considering the scale of the largest private pharmaceutical company in Russia (R–Pharm JSC) and at least one company within Bayer (Germany), we see that the leader of the Russian industry is tiny in comparison (see Tab. 1). If we aggregate all the assets of public and private pharmaceutical companies in Russia (Gusev, Yurevich, 2023), then according to financial indicators their size will remain five times smaller than that of Bayer, but maybe it will approach the parameters of Teva (Israeli company).
Second, the public pharmaceutical sector does not have sufficient resources to innovatively ensure a qualitative shift in at least one segment of pharmaceutical development.
In addition to drugs, there is an area of high–tech developments of biomedical cell products (BMCP), for which separate regulation has been provided in Russia since 2016 [14]. We should note that since 2021 a clinical study of the first and so far the only BMCP from the Russian developer Generium JSC has been conducted: BMCP “Spheroids of human autologous matrix–associated chondrocytes”. Thus, there are no qualitative changes in the field of high–tech developments, which include BMCP.
Conclusion
Import substitution of drugs, which has been officially going on for more than 10 years, is latent. Currently, on the basis of open data, it is not possible to make unambiguous assessments concerning the achievements in this area due to the lack of significant production and marketing information.
The analysis allows us to conclude that, according to formal signs, the vector of outstripping development in the Russian pharmaceutical industry is much inferior to the vector of its catching–up development, and is largely nominal. The implementation of state import substitution plans for pharmaceutical products, which, as a rule, are based on centralized procurement of drugs and their widespread use, remains a priority for participating companies. Outstripping development of pharmaceutical production will not be launched in full until the majority of import substitution tasks are solved. The question of the timing of their solution by the current composition of domestic pharmaceutical companies remains open.
In general, both the catching–up scenario and the outstripping scenario that assumes the creation of a sufficient number of original drugs that surpass existing foreign analogues, involve large–scale and long–term investments, a long planning horizon, consolidation of resources and a mobilization mode of operation. On the basis of self–determination and self–regulation within the domestic pharmaceutical industry, such a strategy is not feasible. It will require state participation and the construction of state–controlled industry innovation giants capable of competing with Big Pharma, at least in the domestic market. One of the institutional scenarios for the mobilization of the industry, based on the creation of Rospharma, the state corporation for pharmaceutical activities, was proposed in the work (Gusev, Yurevich, 2023).
The vertically integrated model of the domestic pharmaceutical industry can be considered as a starting point in its new configuration. In order to comply with the market environment and the structure of manufacturers, this concept can be improved and developed in terms of conditions for the involvement of private companies in the orbit of Rospharma State Corporation, and the allocation of tasks and public resources. In order to promote the scientific and production potential, which for some reason remains outside the contour of the corporation, it seems advisable to consider the formation of state funds to support innovative pharmaceutical developments. We should emphasize that only large–scale and decisive actions in the industry can set it up to achieve technological self–sufficiency and leadership in the long term, and in the medium term – at least in certain sectors.
References
Abdikeev N.M. (2022). Implementation of plans for import substitution in high–tech sectors of domestic industry in the context of external sanctions. Nauchnye trudy Vol’nogo ekonomicheskogo obshchestva Rossii, 235(3), 202–214 (in Russian).
Balatsky E.V., Ekimova N.A. (2019). Innovation–technology matrices and national economic development strategies. Upravlenets – The Manager, 10(5), 9–19. DOI: 10.29141/2218–5003–2019–10–5–2 (in Russian).
Balatsky E.V., Ekimova N.A. (2023). Antifragility of the national economy: A heuristic assessment. Journal of New Economy, 24(2), 28–49. DOI: 10.29141/2658– 5081–2023–24–2–2
Bereznoy A.V. (2022). Transformation of Big Pharma business models. Mirovaya ekonomika i mezhdunarodnye otnosheniya=World Economy and International Relations, 66(3), 81–89. DOI: 10.20542/0131–2227–2022–663–81–89 (in Russian).
Crespi F., Caravella S., Menghini M., Salvatori C. (2021). European technological sovereignty: An emerging framework for policy strategy. Intereconomics, 56(6), 348–354.
DiMasi J.A., Grabowski H.G., Hansen R.W (2016). Innovation in the pharmaceutical industry: new estimates of R&D costs. Journal of Health Economics, 47, 20–33. DOI: 10.1016/j.jhealeco.2016.01.012
Dorzhieva V.V. (2022). State policy of import substitution as a factor in the development of the pharmaceutical industry in Russia: Impact of sanctions and steps to success. VestnikInstituta ekonomiki Rossiiskoi akademii nauk, 6, 68–78. DOI: 10.52180/2073–6487_2022_6_68_78 (in Russian).
Dorzhieva V.V. (2023). The strategy of the new industrialization of the pharmaceutical industry: national priorities and new challenges. Nauchnye trudy Vol’nogo ekonomicheskogo obshchestva Rossii, 240(2), 198–215. DOI: 10.38197/2072–2060–2023–240–2–198–215 (in Russian).
Dosi G., Marengo L., Staccioli J., Virgillito M.E. (2023). Big Pharma and monopoly capitalism: A long–term view. Structural Change and Economic Dynamics, 65, 15–35. DOI: https://doi.org/10.1016/j.strueco.2023.01.004
Groshkova T., Liem M., Cunningham A., Sedefov R., Griffiths P. (2021). Drug–related violence: Will COVID–19 drive better data for safer and more secure EU? The International Journal on Drug Policy, 93, 103143. DOI: 10.1016/j.drugpo.2021.103143
Gulin K.A., Mazilov E.A., Ermolov A.P. (2015). Import substitution as a tool to enhance social–economic development of territories. Problemy razvitiya territorii=Problems of Territory’s Development, 3, 7–25 (in Russian).
Gusev A.B., Yurevich M.A. (2023). The sovereignty of Russia in the area of pharmaceuticals: Challenges and opportunities. Terra Economicus, 21(3), 6–31. DOI: 10.18522/2073–6606–2023–21–3–6–31 (in Russian).
Keenan L., Monteath T., Wojcik D. (2023). Patents over patients? Exploring the variegated financialization of the pharmaceuticals industry through mergers and acquisitions. Competition & Change, 27(3–4), 472–494. DOI: 10.1177/1024529422110785
Komarova A.V., Petrov A.M. (2016). The strategy of import substitution as a factor in increasing the competitive ness of pharmaceutical companies. Rossiiskii vneshneekonomicheskii vestnik=Russian Foreign Economic Journal, 4, 51–62 (in Russian).
Kotlyarova S.N., Lavrikova Yu.G., Averina L.M. (2017). The role of industrial production localization in the import substitution policy. Ekonomicheskie i sotsial’nye peremeny: fakty, tendentsii, prognoz=Economic and Social Changes: Facts, Trends, Forecast, 53(5), 115–127. DOI: 10.15838/esc.2017.5.53.8 (in Russian).
Krestyaninov N.A. (2018). Analysis of government incentives for investments in the Russian pharmaceutical industry. Ekonomika: vchera, segodnya, zavtra=Economics: Yesterday, Today and Tomorrow, 8(8A), 63–77 (in Russian).
Kruse S., Slomiany M., Bitar R. et al. (2014). Pharmaceutical R&D productivity: the role of alliance. Journal of Commercial Biotechnology, 20(2), 11–20. DOI: 10.5912/jcb632
Litvinova A.V., Talalaeva N.S., Parfenova M.V. (2019). Development of methodological approaches to assessing the effectiveness of import substitution in Russia. Ekonomicheskie i sotsial’nye peremeny: fakty, tendentsii, prognoz=Economic and Social Changes: Facts, Trends, Forecast, 12(4), 67–85. DOI: 10.15838/esc.2019.4.64.5 (in Russian).
Mamedyarov Z.A. (2017). Current trends and prospects of the Russian pharmaceutical industry and the foreign experience. MIR (Modernizatsiya. Innovatsii. Razvitie)=MIR (Modernization. Innovation. Research), 8(4), 772–780. DOI: 10.18184/2079–4665.2017.8.4.772–780 (in Russian).
Meier C., Cairns–Smith S., Schulze U. (2013). Can emerging drug classes improve R&D productivity. Drug Discovery Today, 18, 607–609. DOI: 10.1016/j.drudis.2013.05.006
Pammolli F., Magazzini L., Riccaboni M. (2011). The productivity crisis in pharmaceutical R&D. Nature Reviews Drug Discovery, 10(6), 428–438. DOI: 10.1038/nrd3405
Pammolli F., Righetto L., Abrignani S. et al. (2020). The endless frontier? The recent in–crease of R&D productivity in pharmaceuticals. Journal of Translational Medicine, 18, 1–14. DOI: 10.1186/s12967–020–02313–z
Park J.J., Grais R.F., Taljaard M. et al. (2021). Urgently seeking efficiency and sustainability of clinical trials in global health. The Lancet Global Health, 9(5), e681–e690. DOI: 10.1016/S2214–109X(20)30539–8
Paul S.M., Mytelka D.S., Dunwiddie C.T. et al. (2010). How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nature Reviews Drug Discovery, 9(3), 203–214. DOI: 10.1038/nrd3078
Redit C. (2022). Pharma backs off biotech acquisitions. Nature Biotechnology, 40, 1546–1550. DOI: 10.1038/s41587– 022–01529–2
Schuhmacher A., Hinder M., Stein A.V.S. et al. (2023). Analysis of pharma R&D productivity – a new perspective needed. Drug Discovery Today, 103726. DOI: 10.1016/j.drudis.2023.103726
Vargason A.M., Anselmo A.C., Mitragotri S. (2021). The evolution of commercial drug delivery technologies. Nature Biomedical Engineering, 5(9), 951–967. DOI: 10.1038/s41551–021–00698–w
Weigmann K. (2015). The ethics of global clinical trials. EMBO Reports, 16(5), 566–570. DOI: 10.15252/ embr.201540398
Wouters O.J., McKee M., Luyten J. (2020). Estimated research and development investment needed to bring a new medicine to market, 2009–2018. Jama, 323(9), 844–853. DOI: 10.1001/jama.2020.1166
[1] RF Government Resolution 305, dated April 15, 2014.
[2] Drug self–sufficiency is understood as a situation when Russian–made full–cycle drugs (including production of active pharmaceutical ingredients), which are competitive with their foreign counterparts, cover at least 90% the domestic market by volume of consumption (in physical and value terms).
[3] Pharmaceutical leadership is understood as the pace of development of original drugs (new in the world) in number and in the context of areas of application, comparable to similar indicators for countries with developed pharmaceutical industry (USA, UK, Germany, Switzerland).
[4] Import substitution is a tool to ensure drug self–sufficiency. According to the authors, import substitution is considered successful when the condition of achieving at least a 90% share of domestic drugs in the domestic market (in physical and value terms of consumption) is implemented.
[5] According to Federal Law 61-FZ “On circulation of drugs”, dated April 12, 2010, an original medicinal product means a medicinal product with a new active ingredient, which was first registered in the Russian Federation or in foreign countries based on the results of preclinical trials of medicinal products and clinical trials of medicinal products confirming its quality, efficacy and safety.
[6] RF Government Resolution 1495–r, dated June 7, 2023.
[7] HBM (2019). New Drug Approval Report 2019. Available at: https://www.hbmpartners.com/media/docs/industry–reports/Analysis–of–FDA–Approvals–2018–and–Previous–Years.pdf
[8] HBM (2023). Pharma/Biotech M&A Report. Available at: https://www.hbmpartners.com/media/docs/HBM–M–A–Report/HBM–Biopharma–M–A–Report–2022.pdf
[9] Pharmprom. The volume of imports of pharmaceutical ingredients into Russia is growing more and more every year. Available at: https://pharmprom.ru/obyom–importa–farmsubstancij–v–rossiyu–s–kazhdym–godom–rastet–vse–bolshe–i–bolshe/
[10] Abiraterone, Alirocumab, Apixaban, Aflibercept, Buprenorphine, Dapagliflozin, Daratumumab, Dimethyl–fumarate, Dolutegravir, Ibrutinib, Cabazitaxel, Maraviroc, Mitotane, Nimodipine, Nonacog alfa, Omalizumab, Pazopanib, Panitumumab, Pembrolizumab, Pertuzumab, Rilpivirine + Tenofovir + Emricitabine, Tenecteplase, Teriflunomide, Ticagrelor, Trastuzumab emtansine, Tumor necrosis factor alpha–1, Elsulfavirin, Empagliflozin, Eribulin.
[11] Import substitution plans of the Ministry of Industry and Trade of Russia cover 584 drugs from the list of VEDs.
[12] Pharmprom. A list of about a hundred drugs that are at risk of shortage has been issued. Available at: https://pharmprom.ru/defektura–lekarstvennyx–sredstv–spisok/
[13] Levodopa + Benserazide (antiparkinsonian agent); Cisplatin (antitumor drug); Asparaginase (antitumor drug); Rabies immunoglobulin; Tetanus immunoglobulin; Doxycycline (antibiotic); Captopril (angiotensin–converting enzyme inhibitor); Loperamide (antidiarrheal agent); Hydroxyethyl starch (substitute for plasma and other blood components); Neostigmine methylsulfate (anticholinesterase agent); Cetrorelix (antigonadotropic agent); Amoxicillin + Clavulanic acid (semi–synthetic antibiotic)
[14] Federal Law 180–FZ, dated June 23, 2016 “On biomedical cell products”.
Official link to the paper:
Gusev A.B., Yurevich M.A. Catching–up and outstripping development of the Russian pharmaceutical industry // «Economic and Social Changes: Facts, Trends, Forecast», 2023, Vol. 16, No. 6, p. 55–73.